This paper briefly outlines contemporary advancements in hydrogen fuel (HF) quality assurance, focusing on the development of traceable calibration standards, compact analytical instrumentation, and refined on-site sampling protocols.
Latest Advances in Hydrogen Fuel Technologies
Dr. Raj Shah, Dr. Vikram Mittal and Mr. Bob Fang | Koehler Instrument Company
Introduction
Hydrogen fuel cell (HFC) technologies demand ultra-pure hydrogen to operate efficiently and reliably. Trace impurities, including CO, H₂S, and NH₃, can degrade catalyst layers, impair membrane performance, and reduce overall fuel cell longevity even at sub-ppm levels [1, 2, 3]. As such, ensuring hydrogen purity at production and distribution stages has thus become a critical priority for global decarbonization efforts.
This paper briefly outlines contemporary advancements in hydrogen fuel (HF) quality assurance, focusing on the development of traceable calibration standards, compact analytical instrumentation, and refined on-site sampling protocols. Emphasis is placed on compliance with the International Organization for Standardization (ISO) which specifies strict impurity thresholds [4]. ISO 14687:2019 is the standard specifically pertaining to maximum allowable hydrogen impurity levels; the limits are shown below in Table 1. Recent innovations include gravimetrically prepared gas standards, compact chromatography instruments, and quartz-enhanced photoacoustic sensors. Furthermore, inter-laboratory comparisons highlight the variability in analytical performance, reinforcing the need for standardization and metrological harmonization. Addressing these scientific and procedural gaps is essential for scaling hydrogen infrastructure safely and effectively.
Table 1: Maximum concentrations of hydrogen impurities set by ISO 14687:2019 [16]
|
ISO 14687:2019 Component |
Max. impurity level (μmol/mol) |
||
|
Water (H2O) |
5 |
||
|
Total hydrocarbons (except CH4) |
2 |
||
|
Methane (CH4) |
100 |
||
|
Oxygen (O2) |
5 |
||
|
Helium (He) |
300 |
||
|
Argon (Ar) |
300 |
||
|
Nitrogen (N2) |
300 |
||
|
Carbon dioxide (CO2) |
2 |
||
|
Carbon monoxide (CO) |
0.2 |
||
|
Total sulphur compounds (H2S equivalent) |
0.004 |
||
|
Formaldehyde (CH2O) |
0.2 |
||
|
Formic acid (CH2O2) |
0.2 |
||
|
Ammonia (NH3) |
0.1 |
||
|
Halogenated compounds |
0.05 |
||
|
Particulate matter (mg/kg) |
1 |
Current Sensors and Sampling Protocols
There are several sensors and sampling protocols presently employed at commercial hydrogen refueling stations (HRS).
With regards to sensor technology, one of the most popular methods is Optical Feedback Cavity Enhanced Absorption Spectroscopy (OFCEAS). By enclosing a gas sample in a cavity made of highly reflective mirrors, a laser’s adsorption distance can be stretched to several kilometers within a relatively compact device. This technology is notably sold by spectrometry company Proceas, whose H2 purity analyzer can reach sub ppb detection limits. Other sensing instruments include photoacoustic spectroscopy (sold as Multisense by mirSense), mass spectrometry (Protea ProMASS), and gas chromatography (Chromatotech chroma TCD). These methods can all reach up sub ppb detection limits; however, they only detect a handful of designated impurities. save for mass spectrometry, which can detect nearly any gas. [21]
In addition to the array of sensing technologies, there are also several different sampling protocols employed by various HF companies. Because HF is sensitive to impurities, even the action of taking a measurement can pose the risk of contamination if performed improperly. The two main methods of sampling are gas parallel and serial. In the parallel method, a T connector diverts part of the hydrogen stream into a sampling cylinder, which allows simultaneous measurement and vehicle refuelling. In the serial method, hydrogen flows directly into the sampling system. In both gas parallel and serial, the nozzle must be purged repeatedly to avoid cross contamination. Schematics for the aforementioned gas sampling methods are shown below in Figures 1 and 2. Alternatively, hydrogen analysis can be performed off-site by first capturing the impurities via thermal desorption tubes (solid state sorbent sampling) or bubbling the hydrogen gas through water to catch polar and ionic impurities (liquid sampling). Once the contaminants have been captured, they are then sent to a laboratory for further analysis [18, 19].
.jpg)
Figure 1: Example schematic for the serial method of sampling [16].
.jpg)
Figure 2: Example schematic for parallel sampling [16].
New Reference Materials
Because hydrogen fuels demand near perfect purity, high quality reference materials are difficult to synthesize and thus commercially unavailable. Reference materials are samples of hydrogen containing a designated level of impurity. Researchers use these references to calibrate testing instruments and compare results between laboratories. However, maintaining hydrogen fuel to the stringent standards of ISO 14687:2019 is already difficult, and reference gas samples must meet even stricter standards of uncertainty throughout a prolonged shelf life.
Though certified reference materials (CRMs) were produced in Europe as early as 2018, such standards remain scarce internationally at relevant volumes [5].
Lee et al. (2022) addressed the lack of traceable, high-precision gas standards in South Korea [6]. Over the course of 3 years, the authors monitored a series of gravimetrically prepared gas standards containing seven common HF impurities: CO, CO₂, CH₄, O₂, N₂, Ar, and He. These standards were created through a three-step dilution hierarchy using ultra-pure hydrogen and precise automated weighing equipment, with a resolution of 1 mg. The raw gases were pre-screened for impurities using gas chromatography (GC) with multiple detectors, and their concentrations were accounted for in the final uncertainty budgets. The gas mixtures were designed to span the concentration ranges prescribed in ISO 14687:2019 Grade D. The expanded uncertainties achieved were 0.8% for Ar, N₂, and He; 1.0% for CH₄ and CO; and 2.0% for CO₂ and O₂. Verification tests comparing detector sensitivity across standard cylinders showed high consistency, with one outlier excluded. Additionally, the stability of CO in H₂ was assessed over three years, showing only 0.5% variation. The standards were successfully applied to analyze HF from a commercial refueling station, confirming ISO compliance and demonstrating practical utility.
This study delivers the first traceable, high-precision gas standards in Korea that are directly aligned with ISO 14687:2019 HF quality criteria. Increased production of CRM-like standards help coordinate HF quality advancements across various institutions. The developed standards enable reliable calibration of analytical instruments used in hydrogen refueling stations and production facilities. Furthermore, the demonstrated long-term stability and low uncertainty of these standards support their integration into international metrological frameworks.
Future challenges include extending the range of certified standards to encompass additional reactive or low-threshold impurities such as formaldehyde, H₂S, and NH₃, which require more advanced stabilization and detection strategies.
Moreover, though researchers in Europe and Korea are creating reference hydrogen samples, there has been little to no effort in producing reference materials on a commercial scale. Research in high quality reference hydrogen is even more limited outside of those locations. The creation of stable, pure hydrogen reference samples is currently a great obstacle to international standardization and interlaboratory research.
Improvements in Instrumentations
Compaction of Gas Chromatography Instruments
Most of the HF quality assurance today is performed via spectroscopy and, more commonly, chromatography instruments [7, 8, 9]. Conventional offline analytical methods are time-consuming, costly, and unsuited to real-time monitoring at hydrogen refueling stations [10]. Chromatography instruments especially are large and unportable, making them less suitable for sampling at multiple locations. Much of recent research in the field of gas chromatography (GC) sampling is aimed at miniaturizing instrumentation and bringing them online.
D. Kim et al. (2024) addressed the need for a compact, accurate, and field-deployable online analyzer capable of continuously detecting critical impurities in HF [11]. The researchers developed a hydrogen quality analyzer (HQA) integrating GC with a pulsed discharge helium ionization detector (PDHID) for real-time impurity monitoring. Their schematic for their HF analyzer is shown below in Figure 3.
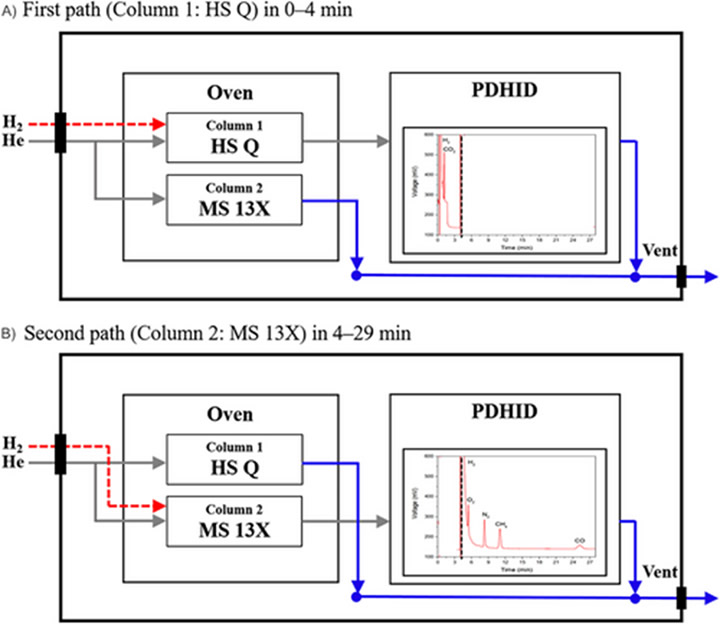
Figure 3: A schematic of the GC-PHID device. The red dashed lines indicate the injected H2 sample, whereas the grey and blue lines respectively indicate He carrier flow and gas flow from the analyzer. The first pathway detects CO2 while the second shows other impurities (O2, N2, CH4, and CO). [11]
The analyzer features a dual-column configuration — HayeSep Q and Molecular Sieve 13X — in a compact oven (60 °C), enabling the separation of CO₂, O₂, N₂, CH₄, CO, and H₂S within 30 minutes. Optimized conditions included 1 mL sample loop, 100 mL/min sample flow, and 60 psi helium carrier gas.
The system exhibited combined analytical uncertainties of 0.21–1.01%, demonstrating strong precision and stability. Calibration used reference gas mixtures with impurity concentrations from ~10 to 20 µmol/mol, achieving linear detector responses (R² ≈ 1.00) and relative standard deviations (RSDs) below 1.1%. Limits of detection (LOD) ranged from 1.4 to 19.0 nmol/mol; limits of quantification (LOQ) ranged from 4.7 to 63.4 nmol/mol. One unique aspect of D. Kim et al.’s work is their use of GC to analyze sulfur-based contaminants like H2S, rather than chemiluminescence, which is recommended by the ISO-21087:2019. Chemiluminescence instrumentation, though accurate, is incompatible with real-time analysis, as it is both costly and inefficient at larger scales. Though this H₂S analysis in N2 showed successful quick separation (elution at 4.5 min) from CO₂, its sensitivity requires further enhancement to meet ISO standards.
Overall, this unique GC–PDHID HQA provides µmol/mol-level detection of key impurities without enrichment or pretreatment, confirming its potential for in situ hydrogen quality monitoring. While the HQA achieves promising precision and sensitivity, improvements are needed to meet the ISO 14687 threshold for sulfur-based contaminants like H₂S. Enhancing detection limits, potentially via larger sample loops or modified switching timing, is essential. Further validation in operational hydrogen refueling environments is required to assess robustness and drift under real-world conditions. Integration of explosion-proof design and broader impurity detection (e.g., ammonia, formaldehyde) will also be necessary. Ultimately, regulatory alignment and certified reference materials will support global deployment of online analyzers for continuous, autonomous HF quality assurance.
S.W. Kim et al. (2023) is another recent paper aimed at miniaturized GC instrumentation, focusing on critical contaminants such as O₂, Ar, N₂, and CH₄ [12]. The researchers developed a compact hydrogen impurity analyzer (HIA) comprising a miniaturized mobile gas chromatograph with a mobile GC thermal conductivity detector (MGT), a hydrogen impurity enrichment device (HIED), and an oxygen sensor. The MGT was optimized to 25% the size of commercial GCs and tailored for selective impurity separation using fixed column configurations.
The schematic for their analyzer is shown below in Figure 4.
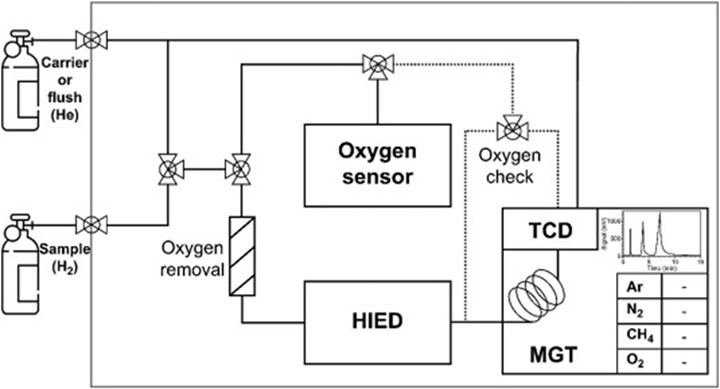
Figure 4: The schematic for S.W. Kim et al.’s (2023) HF analyzer featuring 2 manual threeway valves. This enables 2 main pathways — the first through the oxygen sensor and the latter through HIED and MGT. In the latter pathway, the sample undergoes oxygen removal to account for enrichment error. [12]
Experimental validation with certified reference materials demonstrated that the HIA could detect O₂, Ar, N₂, and CH₄ with LODs of 2.93, 0.72, 0.84, and 1.54 mmol/mol, respectively. Expanded measurement uncertainties were 4.0% for O₂ and approximately 10% for the other analytes — well within ISO 14687 thresholds. Moreover, the added HIEDs increased detection sensitivity by approximately a factor of 15. The researchers’ repeatability and linearity assessments confirmed their system’s robustness. Three enrichment experiments showed high correlation between measured and predicted enrichment factors, validating both analytical precision and the design’s suitability for real-time HF monitoring.
Future development must address the high uncertainty contributions from the minimized oven’s thermal instability, which impacts detector signal consistency. Further improvements could involve stabilizing oven temperatures, integrating more sensitive detectors such as PDHID or FID, and automating system control to reduce manual intervention. Additionally, expanding the analyzer’s capability to include a broader range of impurities beyond O₂, Ar, N₂, and CH₄ (without significantly increasing system size) remains a key challenge. As no existing online analyzer can comprehensively quantify all ISO 14687-specified impurities, modular and hybrid designs will be crucial for broader deployment in hydrogen infrastructure.
Spectrophone: A Novel Detection Method
Feng et al. (2025) created a novel method to detect ammonia in HFCs [13]. Ammonia is a potent impurity because it can degrade HFC performance at only 13 ppm after 1h. Exposure for 15h at 30 ppm irreversibly renders the fuel cell unusable [14]. Currently, NH3 is detected via FTIR, semiconductors, and GC methods, which are bulky and run the risk of corrosion. Recent research in other spectroscopy methods, such as laser-based optics and cavity ring-down, improved the detection of ammonia in hydrogen matrices. A new method called QEPAS exploits ammonia’s photoacoustic effect, where light whose frequency matches that of NH3’s radiative transition causes molecules to release energy into their surrounding H2 matrix. This energy manifests as sound waves, which can be detected by a spectrophone, a quartz tuning fork and a pair of resonance tubes immersed in the gaseous solution.
Experimental validation demonstrated the ability to detect NH3 at 95 ppb with an integration time of 0.1s, well within ISO thresholds. Allowing increased integration time of only 30s could achieve detection limits as low as 1.5 ppb.
Future research must address miniaturization without compromising sensitivity, particularly under varying environmental conditions. One major challenge is maintaining the resonance stability of the QTF in field deployments, where temperature fluctuations and mechanical vibrations may degrade performance. Additionally, integrating this spectrophone into multi-gas detection systems (for the detection of other hydrogen impurities like methane and nitrogen) remains open engineering problems. Further exploration of tuning fork geometries and materials, alongside machine learning-assisted signal processing, could improve detection in complex gas mixtures.
The Continued Need for Collaboration
Though sampling strategies are being shared, especially by European commercial companies, consistent hydrogen impurity detection remains a challenge among researchers. Arrhenius et al. (2024) reports a comprehensive inter-laboratory comparison (ILC) involving 13 laboratories assessing hydrogen fuel quality for eight ISO 14687-relevant contaminants. Gravimetrically prepared high-pressure hydrogen gas mixtures were distributed, with participants applying up to eight analytical techniques (e.g., thermal-conductivity detector GC, PDHID, and mass spectrometry).
Zeta-score analysis revealed satisfactory performance for CO₂ and propane, but significant dispersion for sulfur species and halogenated compounds due to calibrant unavailability and adsorption effects. An example of poor interlaboratory agreement for sulfur impurities is shown below in Figure 5. The study underscores persistent challenges in trace-level impurity quantification, especially for reactive species. Corrective actions post-assessment improved measurement concordance, emphasizing ILCs' role in metrological validation, uncertainty quantification, and international method harmonization for hydrogen purity compliance.
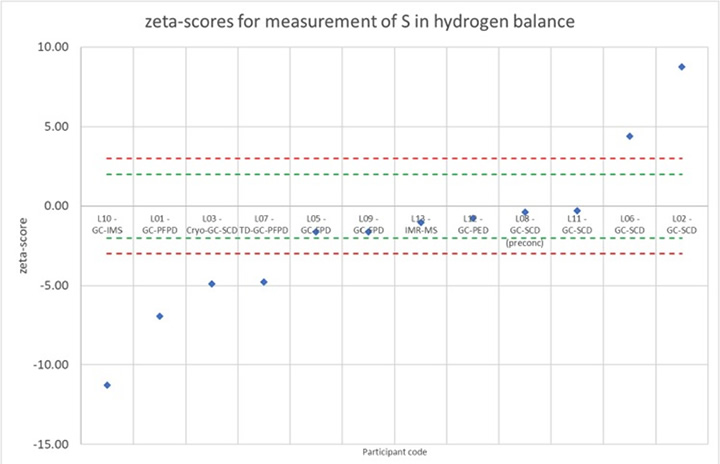
Figure 5: An example of poor agreement between laboratories. A zeta score below 2 is considered satisfactory, which is not the case for sulfur compounds.
Overall, Arrhenius et al.’s work highlights the continued need for collaboration, especially in standardizing HF quality assurance, coordinating corrective actions, and sharing calibrant reference materials.
Conclusion
In summary, hydrogen fuel quality assurance is advancing through the development of traceable calibration standards, miniaturized analytical instrumentation, and refined sampling protocols. Continued international collaboration and standardization are essential to improving accuracy, reducing uncertainty, ultimately enabling enhanced hydrogen purity monitoring critical to the success of hydrogen-based energy infrastructure.
Dr. Raj Shah is a Director at Koehler Instrument Company in New York, where he has worked for the last 25 plus years. He is an elected Fellow by his peers at IChemE, ASTM,AOCS, CMI, STLE, AIC, NLGI, INSTMC, Institute of Physics, The Energy Institute and The Royal Society of Chemistry. An ASTM Eagle award recipient, Dr. Shah recently coedited the bestseller, “Fuels and Lubricants handbook”, details of which are available at ASTM’s Long-awaited Fuels and Lubricants Handbook https://bit.ly/3u2e6GY.
 He earned his doctorate in Chemical Engineering from The Pennsylvania State University and is a Fellow from The Chartered Management Institute, London. Dr. Shah is also a Chartered Scientist with the Science Council, a Chartered Petroleum Engineer with the Energy Institute and a Chartered Engineer with the Engineering council, UK. Dr. Shah was recently granted the honorific of “Eminent engineer” with Tau beta Pi, the largest engineering society in the USA. He is on the Advisory board of directors at Farmingdale university (Mechanical Technology), Auburn Univ (Tribology), SUNY, Farmingdale, (Engineering Management) and State university of NY, Stony Brook (Chemical engineering/ Material Science and engineering). An Adjunct
He earned his doctorate in Chemical Engineering from The Pennsylvania State University and is a Fellow from The Chartered Management Institute, London. Dr. Shah is also a Chartered Scientist with the Science Council, a Chartered Petroleum Engineer with the Energy Institute and a Chartered Engineer with the Engineering council, UK. Dr. Shah was recently granted the honorific of “Eminent engineer” with Tau beta Pi, the largest engineering society in the USA. He is on the Advisory board of directors at Farmingdale university (Mechanical Technology), Auburn Univ (Tribology), SUNY, Farmingdale, (Engineering Management) and State university of NY, Stony Brook (Chemical engineering/ Material Science and engineering). An Adjunct
Professor at the State University of New York, Stony Brook, in the Department of Material Science and Chemical Engineering, Raj also has over 680 publications and has been active in the energy industry for over 3 decades. More information on Raj can be found at https://shorturl.at/JDPZN
Contact: rshah@koehlerinstrument.com
References
1. Wang, X., Baker, P., Zhang, X., Garces, H. F., Bonville, L. J., Pasaogullari, U., & Molter, T.
M. (2014). An experimental overview of the effects of hydrogen impurities on polymer electrolyte membrane fuel cell performance. International Journal of Hydrogen Energy, 39(34), 19701–19713. https://doi.org/10.1016/j.ijhydene.2014.09.151
2. Yuan, X.-Z., Li, H., Yu, Y., Jiang, M., Qian, W., Zhang, S., Wang, H., Wessel, S., & Tommy
T.H. Cheng. (2012). Diagnosis of contamination introduced by ammonia at the cathode in a polymer electrolyte membrane fuel cell. International Journal of Hydrogen Energy, 37(17), 12464–12473. https://doi.org/10.1016/j.ijhydene.2012.05.125
3. Goodwin, J., Zhang, J., Hongsirikarn, K., Colon-Mercado, H., Martinez, M., Greenway, S.,
Finamoore, P., & Deere, J. (2011). Effects of Impurities on Fuel Cell Performance and Durability. https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/review11/fc046_goodwin_2011_p.pdf?sfvrsn=fb2847bc_1
4. (2025). Iso.org. https://www.iso.org/obp/ui/#iso:std:iso:14687:ed-1:v1:en
5. Bacquart, T., Perkins, M., Ferracci, V., Martin, N. A., Resner, K., Ward, M. K. M., Cassidy,
N., Hook, J. B., Brewer, P. J., Irvine, J. T. C., Connor, P. A., & Arul Murugan. (2018). Production and stability of low amount fraction of formaldehyde in hydrogen gas standards. International Journal of Hydrogen Energy, 43(13), 6711–6722. https://doi.org/10.1016/j.ijhydene.2018.02.026
6. Lee, J., Kim, S., & Kim, G. (2022). Preparation of gas standards for quality assurance of
hydrogen fuel. International Journal of Hydrogen Energy, 47(55), 23471–23481. https://doi.org/10.1016/j.ijhydene.2022.05.141
7. Ganzha, V., Ivshin, K., Kammel, P., Kravchenko, P., Kravtsov, P., Petitjean, C., Trofimov, V.,
Vasilyev, A., Vorobyov, A., Vznuzdaev, M., & Wauters, F. (2018). Measurement of trace impurities in ultra pure hydrogen and deuterium at the parts-per-billion level using gas chromatography. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 880, 181–187. https://doi.org/10.1016/j.nima.2017.10.096
8. Meuzelaar, H., Liu, J., Persijn, S., van Wijk, J., & van der Veen, A. M. H. (2020). Trace level
analysis of reactive ISO 14687 impurities in hydrogen fuel using laser-based spectroscopic detection methods. International Journal of Hydrogen Energy, 45(58), 34024–34036. https://doi.org/10.1016/j.ijhydene.2020.09.046
9. A. Murugan, M. de Huu, T. Bacquart, J. van Wijk, K. Arrhenius, I. te Ronde, et al.
Measurement challenges for hydrogen vehicles Int J Hydrogen Energy, 44 (2019), pp. 19326-19333,
10. Arrhenius K, Aarhaug T, Bacquart T, Morris A, Bartlett S, Wagner L, et al. Strategies for the
sampling of hydrogen at refuelling stations for purity assessment. Int J Hydrog Energy. 2021; 46: 34839–34853.
11. Kim, D., Park, M., Kim, S. W., Heo, Y., & Lee, J. (2024). Development of an online
hydrogen fuel quality analyzer with gas chromatography‐pulsed discharge helium ionization detector for applying hydrogen infrastructures. Journal of Separation Science, 47(9-10). https://doi.org/10.1002/jssc.202400088
12. Kim S.W., Beni Adi Trisna, Yin, M., Lim, J., Tae Kyu Ahn, & Lee, J. (2023).
Development of a hydrogen impurity analyzer based on mobile-gas chromatography for online hydrogen fuel monitoring. International Journal of Hydrogen Energy, 48(35), 13012–13023. https://doi.org/10.1016/j.ijhydene.2022.12.233
13. Feng, C., Cui, R., Giansergio Menduni, Zifarelli, A., Pietro Patimisco, Sampaolo, A.,
Spagnolo, V., Dong, L., & Wu, H. (2025). Detection of NH3 impurities in H2 environment exploiting Quartz-Enhanced Photoacoustic Spectroscopy with an optimized spectrophone. Sensors and Actuators B Chemical, 137488–137488. https://doi.org/10.1016/j.snb.2025.137488
14. Uribe, F. A., Gottesfeld, S., & Zawodzinski, T. A. (2002). Effect of Ammonia as Potential
Fuel Impurity on Proton Exchange Membrane Fuel Cell Performance. Journal of the Electrochemical Society, 149(3), A293. https://doi.org/10.1149/1.1447221
15. Publishable Summary for 21GRD05 Met4H2 Metrology for the hydrogen supply chain.
(n.d.). Retrieved May 5, 2025, from https://met4h2.eu/wp-content/uploads/2024/03/21GRD05_M4-Publishable-Summary_Accepted.pdf
16. Chen, Z., Bacquart, T., & Fernicola, V. (n.d.). Review of sampling strategy and requirements
for hydrogen quality analysis Report number Met4H2 -A3.1.1 and A3.1.2 Task 3.1 Activity A3.1.1 and A3.1.2 Reporting date XX Title Review of sampling strategy and requirements for hydrogen quality analysis. Retrieved May 5, 2025, from https://met4h2.eu/wp-content/uploads/2024/12/NPL-report-Sampling-requirements-for-hydrogen-sampling-A3.1.1-and-A3.1.2_rev1.pdf
17. ASTM, “ASTM D7606-11. Standard Practice for Sampling of High Pressure Hydrogen and
Related Fuel Cell Feed Gases.” Dec. 13, 2017. [27]“Sampling from hydrogen refuelling stations,” A4
18. ISO, “ISO 19880-1:2020. Gaseous hydrogen — Fuelling stations — Part 1: General
requirements.” Mar. 2020. [Online]. Available: https://www.iso.org/standard/71940.html
19. Léon, A., & Micero, A. (n.d.). DELIVERABLE REPORT HyQuality Europe - Hydrogen
Quality Assurance in Europe Title: Draft of Emerging Conclusions Lead beneficiary: EIFER. Retrieved May 5, 2025, from https://hyqualityeurope.eu/wp-content/uploads/2024/12/HQE_D5.7-Deliverable-report.pdf
20. Arrhenius, K., Morris, A., Hookham, M., Moore, N., Modugno, P., & Bacquart, T. (2024).
An inter-laboratory comparison between 13 international laboratories for eight components relevant for hydrogen fuel quality assessment. Measurement, 230, 114553–114553. https://doi.org/10.1016/j.measurement.2024.114553
21. Karalikkadan Ashhar, Cui, Y., Kuehsamy, L. J., Wee, D., & Kai, F. M. (2025). A survey of
sensor testing methods for hydrogen leakage detection and purity analysis. Measurement Sensors, 101714–101714. https://doi.org/10.1016/j.measen.2024.101714
The content & opinions in this article are the author’s and do not necessarily represent the views of AltEnergyMag
Comments (0)
This post does not have any comments. Be the first to leave a comment below.
