NREL Scientists Advance Renewable Hydrogen Production Method
Perovskite materials may hold the potential to play an important role in a process to produce hydrogen in a renewable manner, according to an analysis from scientists at the National Renewable Energy Laboratory (NREL).
Hydrogen has emerged as an important carrier to store energy generated by renewable resources, as a substitute for fossil fuels used for transportation, in the production of ammonia, and for other industrial applications. Key to the successful use of hydrogen as a fuel is being able to meet the Department of Energy’s Hydrogen Energy Earthshot—a recently announced goal to cut the cost of clean hydrogen by 80% to $1 per kilogram in a decade.
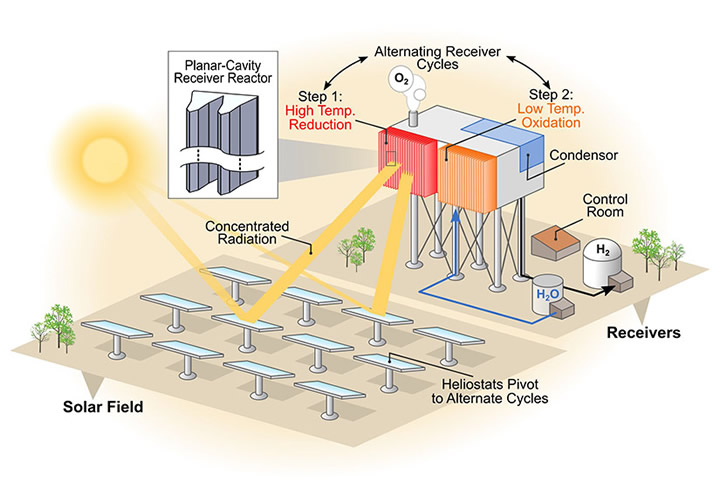
The NREL scientists analyzed an emerging water-splitting technology called solar thermochemical hydrogen (STCH) production, which can be potentially more energy efficient than producing hydrogen via the commonly used electrolysis method. Electrolysis needs electricity to split water into hydrogen and oxygen. STCH relies on a two-step chemical process in which metal oxides are exposed to temperatures greater than 1,400 degrees Celsius and then re-oxidized with steam at lower temperatures to produce hydrogen.
“It’s certainly a very challenging field, and it has a lot of research questions still unanswered, mainly on the materials perspective,” said Zhiwen Ma, a senior engineer at NREL and lead author of a new paper, “System and Technoeconomic Analysis of Solar Thermochemical Hydrogen Production,” which appears in the journal Renewable Energy. His co-authors, all from NREL, are Patrick Davenport and Genevieve Saur.
The paper complements ongoing materials discovery research by looking at the system-level design and techno-economic analysis for integrating possible materials into a solar-fuel platform and supporting the Department of Energy’s HydroGEN program. The material discovery in the HydroGEN program involved machine learning, defect calculations, and experimental work to develop new perovskite materials. The researchers need to identify perovskites capable of handling the high temperatures required while hitting performance targets.
This work shows part of a portfolio of techno-economic analysis focused on hydrogen production pathways each with its own advantages and disadvantages. Electrolysis, for example, is commercially available and the electricity required can come from photovoltaics (PV). The PV cells used, however, only capture a section of the solar spectrum. STCH uses the entire spectrum. The concentrated solar thermal power enables STCH to create the chemical reaction.
Active research to identify the best materials for the STCH process is critical to the success of this method for hydrogen production, the scientists noted.
“The material has not necessarily been found,” Saur said, “but this analysis is to provide some boundaries for where we think the costs will be if the materials meet some of the targets and expectations that the research community envisions.”
This research is funded by the Department of Energy’s Hydrogen and Fuel Cell Technologies Office.
NREL is the U.S. Department of Energy's primary national laboratory for renewable energy and energy efficiency research and development. NREL is operated for the Energy Department by the Alliance for Sustainable Energy LLC.
Comments (0)
This post does not have any comments. Be the first to leave a comment below.
Featured Product

