Beyond Fossil Carbon? Green Electricity Is Opening Doors to Low-Emission Alternatives for Making Fuels and Chemicals

Uncovering Costs, Risks, and Opportunities—NREL researchers, including scientist Zhe Huang (pictured), are analyzing the technical and economic potential of electrifying—and decarbonizing—fuel and chemical production. Photo by Werner Slocum, NREL
Petroleum, coal, and natural gas are not the only starting points for making fuels and chemicals. In fact, growing supplies of renewable electricity open exciting new doors for making identical products at potentially a fraction of the climate cost.
It begins with the steady turn of a wind turbine or a solar panel baking in the mid-afternoon sun. A current flows through an electrochemical cell filled with carbon dioxide (CO2)—siphoned from the air or captured from an ethanol refinery, cement plant, or other industrial source.
Energized by ions and radicals created by the charge, the carbon atom in the gas unglues itself from its oxygen neighbors and looks for new companions to bond with. It quickly latches itself to other newly freed carbon, as well as hydrogen atoms that are generated in the cell.
The exact molecule the carbon helps form depends on the electrocatalyst in the cell and the voltage applied at the outset:
- Formic acid used as a food additive
- Carbon monoxide for making numerous other chemicals
- Ethylene—a precursor in the global plastics market
- And more.
It is an electrochemical reaction, an emerging pathway for upgrading CO2 and even biomass-derived compounds into the many plastics, detergents, fuels, and compounds that undergird the modern economy.
Alongside a broader set of technologies that wield renewable electricity to synthesize chemicals and fuels, the technology holds promise for helping decarbonize heavy industry. But are they really ready for market?
On the Costs, Risks, and Opportunities of Electrifying Chemicals and Fuels Production
“Essentially, we are talking about an intersection of electrification and utilization of low-carbon feedstocks like carbon dioxide and biomass,” said Joshua Schaidle, National Renewable Energy Laboratory (NREL) laboratory program manager for the U.S. Department of Energy’s Office of Fossil Energy and Carbon Management. Schaidle also leads NREL’s catalytic carbon transformation research and directs the U.S. Department of Energy’s Chemical Catalysis for Bioenergy Consortium. “Powered by renewable energy instead of fossil-based electricity, these systems could allow industries to move beyond fossil carbon.”
According to Schaidle and his NREL colleague Gary Grim, that alternative method of making fuels and chemicals could be a critical tool in decarbonizing an economic sector that often leaves deep carbon footprints in its wake.
Instead of dredging up "fossil" carbon stored underground, such methods recycle "modern" carbon found in CO2 or biomass. And rather than relying on carbon-intensive energy sources, they are powered by renewable, zero-emission electricity. The result could be a fuel and chemical production process that is significantly less carbon intensive.
Still, many questions remain about the costs, risks, and technical challenges of making chemicals and fuels from green electricity and recycled carbon. “Where are the technologies at today? Where could they be in the future? And how does that play a role in next steps and future research needs?” Schaidle asked.
In a pair of papers published in Energy and Environmental Science and ACS Energy Letters, Schaidle, Grim, and colleagues explore those questions and others on the technical and economic potential of electrifying—and decarbonizing—fuel and chemical production.
With plenty of uncertainty remaining, they hope doing such work can help mark the path forward from the lab benchtop to the commercial world.
Paper 1: The Economics of Carbon Dioxide Utilization
Studies suggest that technologies exist today for converting CO2 into all the top globally consumed carbon-based chemicals and products—a market currently dominated by fossil sources of carbon.
For example, every year over 10 gigatonnes of carbon is emitted as CO2 around the world. If captured and sent through an electrochemical cell instead, that CO2 can become a feedstock supply—one large enough to produce over 40 times the entire global production of ethylene and propylene.

In an Energy and Environmental Science paper, “The Economic Outlook for Converting CO2 and Electrons to Molecules,” NREL researchers Zhe Huang, Schaidle, Grim, and Ling Tao analyze the economics of electrochemical CO2 utilization today and in the future. The paper considers numerous technology factors and cost drivers that might impact the feasibility of producing chemicals, fuels, and materials from CO2 and renewable electricity at scale.
“We take a broad look across multiple technologies to multiple products,” Grim said. “The key point is that we are using consistent economic assumptions for our analysis.”
According to their study, it could soon be as cost effective to make some of the most widely used chemicals out of CO2 and green electricity as it is to make them using current petroleum-based methods. At the current rate of falling electricity prices and expected improvements in technology, it could even become cheaper in some cases.
“The advancements we are seeing, the activity we are seeing—we will have commercial offerings in the next 5 to 10 years,” Schaidle said. “I think there are opportunities to get down to cost competitiveness, especially as you start to consider any low-carbon credits that come along.”
To arrive at such conclusions, the study incorporates a broad range of assumptions. It considers energy prices and the cost to build new facilities or install new equipment. It factors in technical and chemical influences that could impact the viability of a technology, such as the speed or efficiency of a certain electrochemical reaction.
Not least, the analysis takes a close look at the impact of CO2 source and concentration on the price to make a given chemical, be it carbon monoxide, ethylene, or a hydrocarbon fuel. Where CO2 siphoned directly from the atmosphere is relatively dilute, for example, capturing it from a power plant or biorefinery yields higher concentrations.
To make it easier to sift through the data behind their analysis, Schaidle, Grim, and their colleagues have published a powerful online visualization tool. It includes interactive charts on the economic feasibility and key cost drivers of producing chemical intermediates from CO2 and electricity across five different conversion pathways.
In this way, the takeaways from the paper become easily accessible for a broad audience. For example, their analysis concludes that carbon monoxide made from CO2 and electricity via high-temperature electrolysis—a specific kind of electrochemical technology—would be relatively expensive by today’s standards, at $0.38 per kilogram. Move into the near future, however, and the economics flip. The study projects the price to fall well below today’s market price to $0.15 per kilogram.
“Is this a reality? How close can we get on a cost-competitive basis?” reflected Schaidle. “What are the performers or non-performers?”
With the new paper and visualization tool, arriving at answers is easier than ever before.
Paper 2: The Status of Electrochemical Conversion of Plentiful Biomass
According to the U.S. Department of Energy, biomass resources in the United States could be harnessed to produce up to 50 billion gallons of biofuel each year, more than enough to cover the entire U.S. demand for jet fuel.
But where the carbon in CO2 forms a simple chemical configuration—a gas with one part carbon, two parts oxygen—the renewable carbon in that plentiful biomass is integrated into fibrous networks of lignin and carbohydrates. That makes the starting point for making chemicals with biomass fundamentally different.
Biomass—which includes energy crops, forestry waste, and other organic matter—must first be broken apart into chemical intermediates: polyols, furans, carboxylic acids, amino acids, lignin, and others. Once stored in a more basic form, that renewable carbon can then be more easily accessed, amended, and rearranged.
“You can convert these intermediate molecules thermochemically and biologically, but you can also look at electrochemistry,” Schaidle explained. “Our review focuses on the latter piece, where you are looking at converting an intermediate into a product rather than starting with whole biomass.”
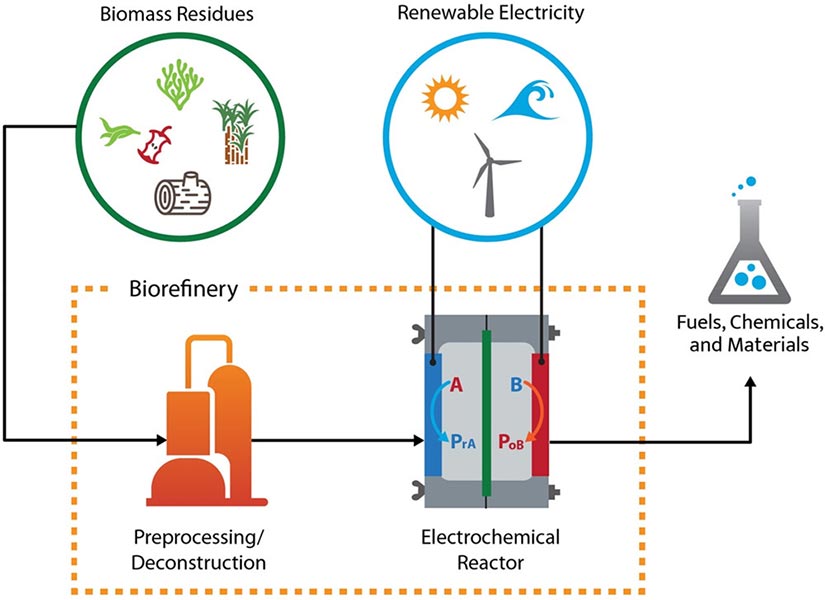
In a second paper published in ACS Energy Letters, Schaidle, Grim, and a larger team of scientists—including Francisco W.S. Lucas and Adam Holewinski from the University of Colorado, Boulder—analyze over 82 reactions driven by the electrochemical synthesis of biomass intermediates. Those reactions have potential advantages, according to the paper.
“Conventional methods only have heat and pressure as their hammers,” Grim explained. “With electrochemistry and biomass intermediates, we have the ability to target specific chemical bonds or groups that can be otherwise difficult to access.”
Grim said that could give industries more latitude to invent chemistries otherwise hard to achieve—a potential advantage over conventional, petroleum-based refining. Still, the electrochemical synthesis of biomass intermediates is immature compared to CO2 utilization.
“If you want this technology to get closer to becoming market competitive, you have to have an electrochemical process that is overall more efficient,” Schaidle added. “It makes the best utilization of the carbon coming in and the best utilization of the electrons coming in. That is where a lot of the technology advancements need to happen.”
By pulling together over 500 publications on the field—articles often focused on specific reactions using electrochemistry—the paper serves as a roadmap for assessing the state of electrochemistry with biomass-derived intermediates and finding the best entry points for improving the technology. With this broad analysis, the team of scientists aims to foster more focus and intentionality in future research.
“This is cross-cutting analysis to help people move forward,” Schaidle added. “We are synthesizing all the science to give a clear blueprint for strategic research.”
Slow But Steady: Steps To Decarbonizing Chemical Manufacturing
Schaidle and Grim are honest about the challenges ahead. After all, should we even try to electrify biomass conversion? Why convert CO2 and not just capture it and put it underground?
“The short answer is that there are a lot of challenges,” Grim said. “Petroleum- and fossil-based processes have had nearly a century head-start on some of these emerging technologies. Those systems are highly optimized, very well studied—and hydrocarbons have a lot of energy already built in.”
With no energy content whatsoever, CO2 must be pumped with massive amounts of cheap, clean energy to successfully transform it into something usable. Many electrochemical technologies for converting biomass intermediates have yet to be scaled beyond the lab—an essential step for demonstrating the stability, efficiency, and affordability of any bioenergy technology. Not least, robust supply chains of renewable electrons, CO2, and biomass are only just emerging.
“The jury is still out: Is this the best use of that abundant future electricity?” Grim asked. “We are still working to understand if these technologies are the best solution for addressing a lot of our climate issues.”
Despite the challenges, Schaidle and Grim remain optimistic that these technologies can play a critical role in decarbonizing fuel and chemical manufacturing.
Supported by the U.S. Department of Energy Bioenergy Technologies Office, ARPA-E, and other energy programs, a range of targeted research projects are already helping push down the cost and increase the efficacy of such technologies. One NREL-led team, for instance, is exploring how to use electrochemistry to enable biorefineries to recycle waste CO2—increasing fuel yields by as much as 40% and decarbonizing the production of ethanol, as well as lipids.
With a nudge in the right direction, more breakthrough projects could be on the horizon.
“How do we guide this field to collectively accelerate everyone’s work?” Schaidle said. “That’s what we wanted to do—to take this blob of an amoeba and turn it into a foundational first step for people to build off of.”
By gathering all the available data—standardizing it, making it comprehensible, giving it form—they hope they can collapse the timeline for improving the technologies. And with deadlines looming for making meaningful progress to lower climate-warming emissions, accelerating R&D could be just what is needed to start eliminating the weighty carbon footprint of making fuels and chemicals.
Explore NREL’s catalytic carbon transformation research, or use NREL’s Economic Feasibility for CO2 Utilization Data Visualization Tool to analyze key CO2 conversion metrics.
Comments (0)
This post does not have any comments. Be the first to leave a comment below.
Featured Product

